Unactivated halogens in aromatic compound undergo indirect nucleophilic displacement in presence of very strong base, which is clearly different from the SNAr mechanism . The mechanism followed by such reaction is known as Benzyne Mechanism.
The intresting features of benzyne mechanism is that the incoming group does not always take the position vacated by the leaving group. This reactions involved elimination on followed by addition . The intermediate molecule is called Benzyne .
Contains the syllabus(organic,physical and inorganic chemistry),notes,guidelines and solutions of Msc Chemistry 1st year and 2nd year run by Tribhuvan University ,Institute of Science and Technolgy.Nepal.
Monday, August 9, 2010
Sunday, August 1, 2010
Neighbouring Group Participation
Neighbouring group participation or NGP in organic chemistry has been defined by IUPAC as the interaction of a reaction centre with a lone pair of electrons in an atom or the electrons present in a sigma bond or pi bond . When NGP is in operation it is normal for the reaction rate to be increased. It is also possible for the stereochemistry of the reaction to be abnormal (or unexpected) when compared with a normal reaction. While it is possible for neighbouring groups to influence many reactions in organic chemistry (For instance the reaction of a diene such as cyclohex-1,3-diene with maleic anhydride normally gives the endo isomer because of a secondary effect {overlap of the carbonyl group π orbitals with the transition state in the Diels-Alder reaction}) this page is limited to neighbouring group effects seen with carbocations and SN2 reactions.
NGP by heteroatom lone pairs
A classic example of NGP is the reaction of a sulfur or nitrogen mustard with a nucleophile, the rate of reaction is much higher for the sulfur mustard and a nucleophile than it would be for a primary alkyl chloride without a heteroatom.
A classic example of NGP is the reaction of a sulfur or nitrogen mustard with a nucleophile, the rate of reaction is much higher for the sulfur mustard and a nucleophile than it would be for a primary alkyl chloride without a heteroatom.
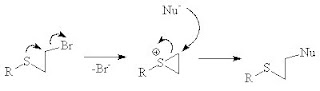
In sugar chemistry anchimeric assistance is an example of NGP.
NGP by an alkene
The π orbitals of an alkene can stabilize a transition state by helping to delocalize the positive charge of the carbocation. For instance the unsaturated tosylate will react more quickly with a nucleophile than the saturated tosylate.
The π orbitals of an alkene can stabilize a transition state by helping to delocalize the positive charge of the carbocation. For instance the unsaturated tosylate will react more quickly with a nucleophile than the saturated tosylate.
The carbocationic intermediate will be stabilized by resonance where the positive charge is spread over several atoms, in the diagram below this is shown.

SN2 Mechanism
The SN2 reaction (also known as bimolecular nucleophilic substitution or as backside attack) is a type of nucleophilic substitution, where a lone pair from a nucleophile attacks an electron deficient electrophilic center and bonds to it, expelling another group called a leaving group. Thus the incoming group replaces the leaving group in one step. Since two reacting species are involved in the slow, rate-determining step of the reaction, this leads to the name bimolecular nucleophilic substitution, or SN2.

Fig:
In an example of the SN2 reaction, the attack of OH− (the nucleophile) on a bromoethane (the electrophile) results in ethanol, with bromide ejected as the leaving group.
SN2 attack occurs if the backside route of attack is not sterically hindered by substituents on the substrate. Therefore this mechanism usually occurs at an unhindered primary carbon centre. If there is steric crowding on the substrate near the leaving group, such as at a tertiary carbon centre, the substitution will involve an SN1 rather than an SN2 mechanism, (an SN1 would also be more likely in this case because a sufficiently stable carbocation intermediary could be formed.)

Fig:
In an example of the SN2 reaction, the attack of OH− (the nucleophile) on a bromoethane (the electrophile) results in ethanol, with bromide ejected as the leaving group.
SN2 attack occurs if the backside route of attack is not sterically hindered by substituents on the substrate. Therefore this mechanism usually occurs at an unhindered primary carbon centre. If there is steric crowding on the substrate near the leaving group, such as at a tertiary carbon centre, the substitution will involve an SN1 rather than an SN2 mechanism, (an SN1 would also be more likely in this case because a sufficiently stable carbocation intermediary could be formed.)
SN1 Mechanism
The SN1 reaction is a substitution reaction in organic chemistry. "SN" stands for nucleophilic substitution and the "1" represents the fact that the rate-determining step is unimolecular.
SN1 reactions take place in two steps (excluding any protonation or deprotonation). The rate determining step is the first step, so the rate of the overall reaction is essentially equal to that of carbocation formation and does not involve the attacking nucleophile.
Thus nucleophilicity is irrelevant and the overall reaction rate depends on the concentration of the reactant only.
rate = k[reactant]
SN1 reactions take place in two steps (excluding any protonation or deprotonation). The rate determining step is the first step, so the rate of the overall reaction is essentially equal to that of carbocation formation and does not involve the attacking nucleophile.
Thus nucleophilicity is irrelevant and the overall reaction rate depends on the concentration of the reactant only.
rate = k[reactant]
Lewis Acid and Bases
Lewis had suggested in 1916 that two atoms are held together in a chemical bond by sharing a pair of electrons. When each atom contributed one electron to the bond it was called a covalentbond.
A Lewis acid, A, is a chemical substance that can accept a pair of electrons from a Lewis base, B, that acts as an electron-pair donor, forming an adduct, AB as given by the following:
A + :B → A—B
Following are some examples of reactions of Lewis acids; acids are the leftmost reactants (e.g. H+):
1.H+ + :NH3 → NH4+
2.Fe3+ + 6 H2O → Fe(III)-(OH)63- + 6 H+ (equilibrium reaction)
3.B2H6 + 2H− → 2BH4−
4. BF3 + F− → BF4−
5. Al2Cl6 + 2Cl− → 2AlCl4−
6.AlF3 + 3F− → AlF63−
7.SiF4 + 2F− → SiF62−
8.PCl5 + Cl− → PCl6−
9.SF4 + F− → SF5−
10.Metal ions forming solvates, such as [Mg(H2O)6]2+, [Al(H2O)6]3+, etc. where the solvent is a Lewis base.
A Lewis base is an atomic or molecular species that has a lone pair of electrons in the HOMO Typical examples are
compounds of N, P, As, Sb and Bi in oxidation state 3
compounds of O, S, Se and Te in oxidation state 2, including water, ethers, ketones, sulphoxides
molecules like carbon monoxide
An easy way to remember this concept is that nearly all of the compounds formed by the transition elements are coordination compounds, wherein the metal or metal ion is a Lewis acid and the ligands are Lewis bases.
A Lewis acid, A, is a chemical substance that can accept a pair of electrons from a Lewis base, B, that acts as an electron-pair donor, forming an adduct, AB as given by the following:
A + :B → A—B
Following are some examples of reactions of Lewis acids; acids are the leftmost reactants (e.g. H+):
1.H+ + :NH3 → NH4+
2.Fe3+ + 6 H2O → Fe(III)-(OH)63- + 6 H+ (equilibrium reaction)
3.B2H6 + 2H− → 2BH4−
4. BF3 + F− → BF4−
5. Al2Cl6 + 2Cl− → 2AlCl4−
6.AlF3 + 3F− → AlF63−
7.SiF4 + 2F− → SiF62−
8.PCl5 + Cl− → PCl6−
9.SF4 + F− → SF5−
10.Metal ions forming solvates, such as [Mg(H2O)6]2+, [Al(H2O)6]3+, etc. where the solvent is a Lewis base.
A Lewis base is an atomic or molecular species that has a lone pair of electrons in the HOMO Typical examples are
compounds of N, P, As, Sb and Bi in oxidation state 3
compounds of O, S, Se and Te in oxidation state 2, including water, ethers, ketones, sulphoxides
molecules like carbon monoxide
An easy way to remember this concept is that nearly all of the compounds formed by the transition elements are coordination compounds, wherein the metal or metal ion is a Lewis acid and the ligands are Lewis bases.
Labels:
Acid and base
Super Acids
A superacid is an acid with an acidity greater than that of 100% pure sulfuric acid, which has a Hammett acidity function (H0) of −12. Commercially available superacids include Trifluoromethanesulfonic acid(CF3SO3H), also known as triflic acid, and Fluorosulfonic acid(FSO3H), both of which are about a thousand times stronger (i.e. have more negative H0 values) than sulfuric acid. The strongest superacids are prepared by the combination of two components, a strong Lewis acid and a strong Brønsted acid. The strongest known superacid is Fluoroantimonic acid.
Labels:
Acid and base
Saturday, July 31, 2010
Hard and Soft acid and Bases
The concept of hard and soft acids and bases.
Soft bases: the donor atoms are of relatively low electronegativity and high
polarizability and are easy to oxidize. High polarizability: they hold their valence
electrons loosely.
Hard bases: the donor atoms are of high electronegativity and low polarizability and
are hard to oxidize. They hold their valence electrons tightly.
Soft acids: the acceptor atoms are large, have low positive charge, and contain unshared
pair of electrons (p or d) in their valence shells. They have high polarizability and low
electronegativity.
Hard acids: the acceptor atoms are small, have high positive charge, and do not contain
unshared pair of electrons in their valence shells. They have low polarizability and high
electronegativity.
Soft bases: the donor atoms are of relatively low electronegativity and high
polarizability and are easy to oxidize. High polarizability: they hold their valence
electrons loosely.
Hard bases: the donor atoms are of high electronegativity and low polarizability and
are hard to oxidize. They hold their valence electrons tightly.
Soft acids: the acceptor atoms are large, have low positive charge, and contain unshared
pair of electrons (p or d) in their valence shells. They have high polarizability and low
electronegativity.
Hard acids: the acceptor atoms are small, have high positive charge, and do not contain
unshared pair of electrons in their valence shells. They have low polarizability and high
electronegativity.
Labels:
Acid and base
Effect of diffrent factors on the strength of Acids and Bases
FIELD EFFECT:
The presence of the electron withdrawing substituents in the residues of acids in general provides stabilization of the carboxylate anion, and therefore enhances the acidity (eg. acetic acid: pKa=4.76, nitroacetic acid: pKa=1.68, fluoroacetic
acid: pKa=2.66).
This effect is, however, general (not limited to carboxyacids). We may say that groups that
withdraw electrons by the field effect increase acidity and decrease basicity, while
electron-donating groups act in the opposite direction.
OR
The effect that operates through the space but not through the bond is called field effect. It usually operates along through the inductive effect so it is difficult to remove or seperate the effect of them but the inductive depends on the nature of bonds.
2. RESONANCE EFFECT: Resonance effects that stabilize bases but not their conjugated
acids result in higher acidity (and vice versa). Example: beta-ketoesters are more acidic
than simple ketones or carboxylic esters.
In general, the electron withdrawing substituents increase acidity and decrease basicity,
while the electron donating groups act in the opposite manner.
OR
The decrease in electronegativity at one position and corresponding increases other place within the molecule due to the movement of pi electron or unshared paired of electron is known as resonance effect.
3. PERIODIC SYSTEM CORRELATION
* acidity increases / basicity decreases going from left to right in the PT (e.g. acidity
increases CH4 <> NH2- > HO- > F-).
*acidity increases / basicity decreases going from top to bottom in the PT (e.g. HF <> PH3 > AsH3).
This is related to the size of the species involved. For example, the small and hard F-
attracts a proton more than large and soft I-.
* acids that require only one electron pair to fill an outer shell are stronger acids than the
ones that need two electron pairs (e.g. GaCl3 is a stronger acid than ZnCl2).
*likewise, the acidity of MXn decreases in going down the PT (the size increases and the
attraction between a positive nucleus and a negative electron pair is weaker). BCl3 is
therefore stronger acid than AlCl3.
3. HYDROGEN BONDING:
Internal hydrogen bonding can greatly effect acidity and basicity. For example, the pKa of
ortho-hydroxybenzoic acid is pKa=2.98, while the pKa of the para-isomer is 4.58. Here the
intramolecular hydrogen bond stabilizes the carboxylate anion.
In this bond the hydrogen atom is bonded with two or more electronegative element. Internal hydrogen bonding can greatly effect the acidity and basicity ...
5. STERIC EFFECT:
The presence of the electron withdrawing substituents in the residues of acids in general provides stabilization of the carboxylate anion, and therefore enhances the acidity (eg. acetic acid: pKa=4.76, nitroacetic acid: pKa=1.68, fluoroacetic
acid: pKa=2.66).
This effect is, however, general (not limited to carboxyacids). We may say that groups that
withdraw electrons by the field effect increase acidity and decrease basicity, while
electron-donating groups act in the opposite direction.
OR
The effect that operates through the space but not through the bond is called field effect. It usually operates along through the inductive effect so it is difficult to remove or seperate the effect of them but the inductive depends on the nature of bonds.
2. RESONANCE EFFECT: Resonance effects that stabilize bases but not their conjugated
acids result in higher acidity (and vice versa). Example: beta-ketoesters are more acidic
than simple ketones or carboxylic esters.
In general, the electron withdrawing substituents increase acidity and decrease basicity,
while the electron donating groups act in the opposite manner.
OR
The decrease in electronegativity at one position and corresponding increases other place within the molecule due to the movement of pi electron or unshared paired of electron is known as resonance effect.
3. PERIODIC SYSTEM CORRELATION
* acidity increases / basicity decreases going from left to right in the PT (e.g. acidity
increases CH4 <> NH2- > HO- > F-).
*acidity increases / basicity decreases going from top to bottom in the PT (e.g. HF <> PH3 > AsH3).
This is related to the size of the species involved. For example, the small and hard F-
attracts a proton more than large and soft I-.
* acids that require only one electron pair to fill an outer shell are stronger acids than the
ones that need two electron pairs (e.g. GaCl3 is a stronger acid than ZnCl2).
*likewise, the acidity of MXn decreases in going down the PT (the size increases and the
attraction between a positive nucleus and a negative electron pair is weaker). BCl3 is
therefore stronger acid than AlCl3.
3. HYDROGEN BONDING:
Internal hydrogen bonding can greatly effect acidity and basicity. For example, the pKa of
ortho-hydroxybenzoic acid is pKa=2.98, while the pKa of the para-isomer is 4.58. Here the
intramolecular hydrogen bond stabilizes the carboxylate anion.
In this bond the hydrogen atom is bonded with two or more electronegative element. Internal hydrogen bonding can greatly effect the acidity and basicity ...
5. STERIC EFFECT:
Labels:
Acid and base
Utility of Hammette Equation
1. I has been very successful in the treatment of the effect of group of para and meta position . It has also been in attempt to apply it to ortho position.
2. σ- determines the effect of Substituents and rho determones the sensitivity of the reactions.
3. Hammett e equation may be applied to many physical measurement including infrared frequency and NMR chemical shift.
4. It is successful to explain whether the substrate is attacked by electrophilic, nuclephilic or free radical reagent.
5. σ and rho value are also applicable for the prediction of electrophilic situation on reaction center,
2. σ- determines the effect of Substituents and rho determones the sensitivity of the reactions.
3. Hammett e equation may be applied to many physical measurement including infrared frequency and NMR chemical shift.
4. It is successful to explain whether the substrate is attacked by electrophilic, nuclephilic or free radical reagent.
5. σ and rho value are also applicable for the prediction of electrophilic situation on reaction center,
Hammette Equation
To give the numerical values of the effect of structure on reactivity the first try was taken by Hammett e and known as Hammette equation .
The basic equation is:
The basic equation is:

relating the equilibrium constant, K, for a given equilibrium reaction with substituent R and the reference K0 constant when R is a hydrogen atom to the substituent constant σ which depends only on the specific substituent R and the reaction constant ρ which depends only on the type of reaction but not on the substituent used.
The equation also holds for reaction rates k of a series of reactions with substituted benzene derivatives:
Wednesday, May 12, 2010
Chemistry
This site brings you the Syllabus solution on few topics in Master Progaramme of Chemistry of Tribhuvan University.
Subscribe to:
Posts (Atom)


